No products
Buy pain reliefers like codein, tilidin and tramadol for biggest effect, safe and secure.
Pain relief No products in this category.
Subcategories
-
Tilidine
1. What is tilidine?
Tilidine is a combination of a powerful analgesic
the group of opioids and an opioid antagonist.2. How to take tilidine?
Take Tilidine
always exactly as instructed by the doctor. You're welcome
Ask your doctor or pharmacist if you are not sure.
Tilidine
is particularly suitable for the treatment of chronic pain. The
required dose and the interval of taking are by the doctor for each patient
determined individually.
The daily dose of Tilidine
can, depending on the level of pain and individual
Response to treatment, between 100 mg and a maximum of 600 mg (based on
Tilidine hydrochloride).
Unless otherwise prescribed by the doctor, the usual dose is:
The usual initial dose of Tilidine
is twice daily 100 mg.
In doing so, a time interval of 12 hours should be kept between the revenues
become.
If you have not previously taken an opioid, your doctor may start with the starting dose of
Tilidine
reduce to 50 mg twice daily.
Is the pain treatment with tilidine comp. 2 times a day 100 mg. STADA®
Not
Sufficient, your doctor will tell you the tilidine
Dosage stepwise
up to one
Increase the dose sufficient
Pain control at
tolerable side effects
achieved.
Tilidine
100 mg / 8 mg is suitable for a daily dose of 200 mg to
400 mg (based on tilidine hydrochloride).
Adults and adolescents from 14 years:
Take 1-2 prolonged-release tablets Tilidine
100 mg / 8 mg
(corresponding to 200 mg or 400 mg tilidine hydrochloride per day).
If other doses are required, Tilidine
50 mg / 4 mg,
Tilidine
150 mg / 12 mg and Tilidine
200 mg / 16 mg as further
Strengths available. All sustained-release tablet strengths can be combined as needed
be combined.
Note
The dosages recommended here are guidelines. In individual cases may be for treatment
very high pain, exceeding the maximum dose and shortening the interval between them will become necessary.
Dosage in renal impairment
Renal impairment does not require a dose change.
Dosage in elderly patients
Dose adjustment is not required in elderly patients.3. Side effects of tilidine?
Like all medicines, Tilidine have side effects, but not everybody gets them. The frequencies for side effects are based on the following categories:
Diseases of the nervous system
Common: Dizziness, drowsiness, fatigue, headache, nervousness.
Uncommon: somnolence.
Unknown: hallucinations, state of confusion, euphoric mood, trembling,
Increased reflex readiness, muscle twitching.
Gastrointestinal disorders
Very common: Nausea and vomiting can very often occur at the start of treatment,
which with further treatment only frequently to occasionally or rarely occur.
Common: diarrhea, abdominal pain.4. How to store tilidine?
Store drug out of reach of children.
You must take the medicine after the carton and blister pack
no longer apply the expiry date.
Do not store above + 30 ° C.
Medicines should not be disposed of in waste water or household waste. Ask your pharmacist how to dispose of the medicine when you no longer need it.
This measure helps to protect the environment. -
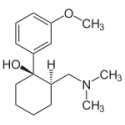
Tramadol
1. What are Tramadol and what is it used for?
Tramadol hydrochloride - the active ingredient in Tramadol - is an analgesic (analgesic) from the group of opioids, which acts on the central nervous system. Its pain relief reaches it by its effect on certain nerve cells of the spinal cord and the brain.
Tramadol are used to treat moderate to severe pain.2. What should you watch out for before taking Tramadol?
Tramadol should not be taken,
If you are allergic to tramadol hydrochloride or any of the other ingredients of this medicine (listed in section 6).
in acute poisoning with alcohol, sleeping pills, analgesics or other psychotropic drugs (drugs with effects on mood and emotional life).
if you are taking MAO inhibitors (medicines to treat depression) or if you have taken drops within the last 14 days prior to treatment with Tramal (see "Taking Tramadol with other medicines").
if you have epilepsy and your seizures can not be adequately controlled by treatment.
as a substitute for drug withdrawal.
Warnings and Precautions:
Please talk to your doctor or pharmacist before you use Tramadol,
if you think you are dependent on other painkillers (opioids) or have already been sensitive to opioids in the past.
if you have a mental disorder (if you feel close to fainting).
when you are in shock (cold sweat may be an indication).
if you have conditions with increased intracranial pressure (possibly after a head injury or brain disease).
if you have difficulty breathing.
if you are prone to epilepsy or seizures.
if you have liver or kidney problems.
Epileptic seizures have been reported in patients taking tramadol at the recommended dose. The risk could increase if the recommended maximum daily dose (400 mg) is exceeded.
Please note that Tramadol can lead to a physical and emotional dependence. With prolonged use, the effect of Tramadol may decrease, so that higher doses (amounts of the drug) must be taken (tolerance development). Patients who are prone to drug abuse or have drug dependence should therefore only receive Tramal treatment at short notice and under the strictest medical supervision.
If a patient discontinues tramadol therapy, especially after prolonged use, a gradual dose reduction is recommended to avoid withdrawal symptoms.
Please also tell your doctor if any of these problems occur while you are taking Tramadol, or if this information was previously available to you.Taking Tramadol with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Tramadol should not be taken with MAO inhibitors (certain medicines used to treat depression).
The analgesic effect of Tramadol can be reduced and the duration of action shortened if you take medicines containing any of the following active ingredients.
Carbamazepine (for epileptic seizures)
Ondansetron (for nausea)
Your doctor will tell you if and if so in what dosage Tramadol may be taken.
The risk of side effects increases when you
Tramadol and sedatives, hypnotics, other painkillers such as Take morphine or codeine (also cough medicine) and alcohol. They may feel drowsy or feel close to fainting. If this happens, please inform your doctor.
Taking medicines that can cause seizures (seizures), such as certain antidepressants or antipsychotics (medicines that affect the consciousness). The risk of having a seizure may be increased if you take Tramal retard. Your doctor will tell you if Tramal retard is right for you.
take certain antidepressants (medicines for depression). Tramal retard may interact with this medicine and you may experience symptoms such as involuntary, rhythmic muscle contractions, including muscle, which control the movement of the eyes, restlessness, excessive sweating, tremors, exaggerated reflexes, increase in muscle tension, body temperature above 38 ° C feel.
concomitantly with Tramadol coumarin anticoagulants (medicines for blood thinning), e.g. Warfarin. The effect of these medicines on blood coagulation may be affected and bleeding may occur.
Taking Tramadol along with food and alcohol
Do not drink alcohol during treatment with Tramadol, as its effects may be increased. Foods do not affect Tramadol.Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
pregnancy
There is limited information on the safety of tramadol hydrochloride in pregnancy. Therefore, you should not take Tramadol during pregnancy.
Prolonged use during pregnancy may lead to withdrawal symptoms in the newborn (the neonate may experience a change in the respiratory rate).
Your doctor will therefore only prescribe Tramadol during pregnancy if you have to.
lactation
Taking tramadol hydrochloride while breast-feeding is generally not recommended. Tramadol hydrochloride is excreted in breast milk in very small amounts. With a single dose, an interruption of breastfeeding is usually not required.
Procreation / childbearing
Based on human experience, tramadol does not affect female or male fertility.
Driving and using machines
CAUTION: This medicine may affect the ability to react and to drive!
Traminal drops can cause drowsiness, dizziness, and blurred vision, and affect your ability to react. If you feel that your responsiveness is impaired, do not drive a car or other vehicle, do not operate electrical tools or machinery.
Tramadol contains sucrose and macrogolglycerol hydroxystearate
If your doctor has told you that you have an intolerance to certain sugars, ask your doctor for advice before taking Tramadol, as the tablets contain sucrose. If Tramadol are intended for chronic use (i.e., for two weeks or longer), they could be detrimental to the teeth.
Tramadol contain macrogolglycerol hydroxystearate, a derivative of castor oil that can cause stomach upset and diarrhea.3. How to take Tramadol?
Always take this medicine exactly as your doctor or pharmacist has told you. Ask your doctor or pharmacist if you are not sure.
The dose should be adjusted to the intensity of your pain and to your individual pain sensation. In general, the smallest possible dose, with which freedom from pain is achieved, should be taken.
Do not take more than 160 drops (for example, 8 times 20 drops) (equivalent to 400 mg tramadol hydrochloride) daily, unless your doctor has ordered it accordingly.
Unless otherwise prescribed by the doctor, the usual dose is:
Adults and adolescents from 12 years
The usual dose of Tramadol in the dropper bottle is 20 to 40 drops (equivalent to 50 to 100 mg of tramadol hydrochloride).
The effect lasts about 4 to 8 hours, depending on the intensity of the pain.
For moderately severe pain, 50 mg tramadol hydrochloride (20 drops) should be administered. If there is no adequate pain relief within 30 to 60 minutes, another 50 mg can be administered. For severe pain conditions, 100 mg tramadol hydrochloride (40 drops) may be given as an initial dose.
Children from 1 year
The usual single dose Tramadol in the dropper bottle for infants from 1 year of age with the dropper is 4 to 8 drops per 10 kg of body weight (corresponding to about 1-2 mg of tramadol hydrochloride per kg of body weight). You will find detailed information about the body weight dose at the end of this leaflet.
In general, the lowest effective dose is chosen to achieve freedom from pain.
Daily doses of 8 mg tramadol hydrochloride per kg body weight or 400 mg tramadol hydrochloride (whichever is lower) should not be exceeded.
For the use of Tramadol in children it is recommended to administer the solution by means of a dropper bottle and not with the aid of a bottle with a dosing pump in order to allow an exact body weight related dosage.
Elderly patients
In elderly patients (over 75 years), excretion of tramadol hydrochloride may be delayed. If this applies to you, your doctor may recommend that you prolong the dose interval.Severe liver or kidney disease (insufficiency) / dialysis patients
If you have liver and / or kidney failure, your doctor may recommend prolonging the dose interval.
How and when should you take Tramadol?
Oral drops (for oral use).
Take the drops with some liquid or on a sugar cube. A detailed description of the use of the dropper bottle can be found at the end of this leaflet. You can take Tramadol on an empty stomach or with a meal.
How long should you take Tramadol?
You should not take Tramadol for longer than absolutely necessary. If, depending on the type and severity of the disease, a longer lasting pain treatment seems to be necessary, your doctor will check at regular intervals (possibly by taking breaks), if you should continue to use Tramadol and if so in which dose.
Please talk to your doctor or pharmacist if you think the effects of Tramadol are too strong or too weak.
If you take more Tramadol than you should
Inadvertently, if you accidentally take an additional dose of Tramadol, it will not have a negative effect. Further intake of Tramadol should be made as prescribed.
Ingestion of very high doses can lead to narrow pupils, vomiting, drop in blood pressure, accelerated heartbeat, circulatory collapse, impaired consciousness, even coma (deep unconsciousness), epileptiform seizures and difficulty breathing, including respiratory arrest. Immediately call a doctor for help when these signs appear!
If you forget to take Tramadol
If you forget to take Tramadol, your pain may recur. Do not take the double dose, but continue as before.
If you stop taking Tramadol
If you stop treatment with Tramadol or stop prematurely, this will lead to a recurrence of the pain. If you would like to discontinue the treatment because of unpleasant side effects, please contact your doctor.
In general, stopping treatment with Tramadol will have no after-effects. In rare cases, however, patients treated with Tramadol for extended periods of time may feel uncomfortable when treatment is stopped abruptly. You may feel restless, anxious, nervous or shaky. You may be hyperactive, have difficulty sleeping or have gastrointestinal discomfort. Very few people may experience panic attacks, cognitive disorders, abnormalities such as itching, tingling and numbness and tinnitus. Other unusual disorders affecting the central nervous system, such as Confusion, delusions, disturbance of the ego experience (depersonalization), disturbance in the perception of reality (derealization) and persecution mania (paranoia) were observed very rarely. If any of these symptoms occur after stopping treatment, contact your doctor.
If you have any further questions on the use of the medicine, ask your doctor or pharmacist.4. What side effects are possible from tramadol?
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You should see a doctor immediately if you experience symptoms of an allergic reaction such as swelling of the face, tongue and / or throat and / or difficulty in swallowing or rash with concomitant breathing difficulties.
The most common side effects that occur during treatment with Tramadol are nausea and dizziness, which is more common than in 1 in 10 people.
Very common: may affect more than 1 in 10 people
dizziness
nausea
Frequently: can affect up to 1 in 10 people
Headache, drowsiness
Constipation, dry mouth, vomiting
Sweating (hyperhidrosis)
exhaustion
Uncommon: may affect up to 1 in 100 people
Influencing the heart activity (palpitations, increased heartbeat). These side effects can occur especially with upright posture and physical stress.
Nausea, stomach discomfort (eg stomach pressure, feeling of fullness), diarrhea
Skin reactions (eg itching, rash)
Rarely: can affect up to 1 in 1000 people
allergic reactions (e.g., difficulty breathing, wheezing, swelling of the skin) and shock (sudden circulatory failure) have occurred in very rare cases
Slowed heartbeat
Increase in blood pressure
Misbehavior (such as itching, tingling sensation, numbness), trembling, epileptic seizures, muscle twitching, coordination disorders, temporary loss of consciousness (fainting), speech disorders
Epileptic seizures occurred predominantly after administration of high doses of tramadol or when tramadol is taken concomitantly with drugs that cause seizures.
appetite changes
Perception disorders (hallucinations), confusion, sleep disorders, delirium, anxiety and nightmares
Mental complaints may occur after treatment with Tramal, with their intensity and nature varying in individual ways (depending on the patient's personality and duration of use). These may be mood changes (usually elevated, occasionally irritable mood), changes in activity (usually attenuation, occasional increase) and reduction in cognitive and sensory performance (change in sensory perception and cognition, which can lead to errors in decision-making behavior) ,
A dependency can occur. If Tramadol are taken over a longer period of time, drug dependency may develop, although the risk is low. With abrupt discontinuation of the drug withdrawal reactions may occur (see "If you stop using Tramadol").
Blurred vision, narrowing of the pupils (miosis), strong pupillary dilation (mydriasis)
Reduction of breathing, shortness of breath (dyspnoea)
An exacerbation of asthma has been reported, but a link with the drug tramadol hydrochloride could not be established. Exceeding the recommended doses or using other medicines that act to depress the brain at the same time may result in decreased breathing.
Decreased muscle power
Difficult or painful urination or less urine than normal (dysuria)
Very rare: may affect up to 1 in 10,000 people
Increase in liver enzyme levels5. How to store Tramadol?
Tramadol are stable after opening at 25 ° C - 30 ° C until the expiry date stated.
Keep this medicine out of the sight and reach of children!
Do not use this medicine after the expiry date which is stated on the carton and the glass bottle label. The expiry date refers to the last day of the specified month.
Do not dispose of medicines in wastewater or household waste. Ask your pharmacist how to dispose of the medicine when you stop using it. You help to protect our environment. -
Codein
1. What are codein and what are they used for?
Codeine-drop CT is a drug for the symptomatic treatment of irritating cough.
Codein are applied
- for the symptomatic treatment of irritating cough (unproductive cough).
2. What do you need to know before taking codein?
Codein should not be taken
- if you are hypersensitive to codeine, sodium disulfite or any of the other ingredients
of Codein are
- if you have functional respiratory (respiratory insufficiency) or respiratory (respiratory
depression)
- if you have an acute asthma attack
- if you have a chronic cough, B. a warning sign for a beginning
Asthma may be bronchial. In particular, it is important to look out for children
- of children under 2 years
- if you are about to give birth
- if you have been diagnosed with a threatening premature birth
- if you are taking certain medicines for depression called MAO inhibitors, such as B.
Moclobemide (see also "Taking codein with other medicinal products
Delivery ")
Take special care with codein
- if you are dependent on opioids
- if you suffer from impaired consciousness
- if you experience disturbances of the respiratory center (eg in conditions associated with increased intracranial pressure).
go forth) and disorders of respiratory function
- if the gallbladder has been removed (condition after cholecystectomy)
- if your cough is accompanied or increased by increased mucus production
other sputum comes. In these cases, codein may only become more stringent
Take benefit-risk consideration by your doctor as it creates a dangerous secretion
can come
- if you are one of the persons suffering from a genetic variant of the liver
enzyme CYP2D6 (polymorphism) convert codeine very rapidly to morphine, since it is in
In this case, a relative overdose can occur.
at higher doses:
- if you suffer from low blood pressure due to lack of fluids
children
This medicine should not be given to children under 2 years of age.
Taking codein with other medicines
Tell your doctor or pharmacist if you are taking / using any other medicines.
or recently used, even if it is not a prescription
medicinal products subject to the duty to pay.
The following drugs may be influenced in their effect by codein, or
in turn, affect the effect of codein. Therefore ask for a similar
In the case of early administration of the following drugs with codeine-drop CT, be sure to consult your doctor.
- sedative and sleep aid
- psychotropic drugs (phenothiazines such as chlorpromazine, thioridazine, perphenazine)
- Agents for the treatment of allergies (antihistamines such as promethazine, meclozin)
- hypotensive agents (antihypertensive agents).
- tricyclic antidepressants such. Imipramine, amitriptyline and opipramol
- certain strong painkillers such. B. buprenorphine, pentacozin (so-called
partial opioid agonists / antagonists)
- cimetidine
MAO inhibitors such. B. Moclobemide:
Codein may not be used until two weeks after the end of MAO inhibitor therapy
be taken.
Taking codein together with food and drink
Do not drink alcohol while using codein. Codeintropfen-
CT, together with alcohol, diminishes the ability to solve complex tasks and tasks
Concentration (psychomotor performance) stronger than the individual substances.
pregnancy and breast feeding period
If you are pregnant, you may only codein on your express order
Doctors take as unwanted effects on the development of the unborn child
can not be excluded.
Do not use codein when you are approaching delivery or imminent premature birth,
because the codeine contained in drug code-codeine happens to pass the placental barrier and the
Newborn can lead to respiratory problems.
For longer term use of codein, a code dependency of the
develop unborn child. Reports of withdrawal symptoms in the newborn after
repeated use of codeine in the last third of pregnancy are present.
Therefore, contact your doctor immediately if you are planning to become pregnant or
are already pregnant to discuss the continuation or conversion of therapy
to advise.
lactation
Codeine and its degradation product morphine are excreted in breast milk.
In general, single use of codein can be performed by your doctor
recommended dose to be breastfed. If your child has drinking difficulties or unusual
calm.3. How to take codein?
At the beginning of treatment, look for any signs of overdose (see "If
You have taken a greater amount of codein than you should "). by virtue of
a genetic variant of the liver isoenzyme CYP2D6 (polymorphism) becomes codeine
Some people convert very quickly to morphine, leading to a relative overdose
can lead.
Always take codein exactly as your doctor has told you. Please ask
Check with your doctor or pharmacist if you are not sure4. What side effects are possible?
Like all medicines, codein can have side effects, although not everybody does
must occur.
The evaluation of side effects is based on the following frequencies:
very often
more than 1 in 10 people treated
frequently
less than 1 in 10, but more than 1 in 100 people
occasionally
less than 1 in 100, but more than 1 in 1000 people
Rare
less than 1 in 1000, but more than 1 in 10,000 people
very rare
less than 1 in 10,000 people, including isolated cases
nervous system
frequently:
mild headache, mild drowsiness
occasionally:
sleep disorders
gastrointestinal tract
very common: nausea, u. U. until vomiting (especially at the beginning of therapy), constipation
(Constipation)
occasionally:
dry mouth
respiratory tract
occasionally:
shortness of breath skin
occasionally:
Itching (pruritus), hives (urticarial exanthema)
Rare:
severe allergic reactions including Stevens-Johnson syndrome
At higher doses or in particularly sensitive patients dose-dependently
Ability to optically fix objects (visuomotor coordination) and impair vision. Also disturbances of the respiratory drive (respiratory depression)
and morbid high spirits (euphoria) occur.
Codeine may, especially in single doses above 60 mg,
tonus) of the involuntary muscles (smooth muscle) z. B. intestinal muscles, increase bladder muscles.
With high therapeutic doses and with poisonings fainting (syncope) and
Hypotension may occur in patients with pre-existing pulmonary dysfunction
the occurrence of pulmonary edema can be expected.
Other possible side effects
Sodium disulfite rarely causes hypersensitivity reactions and bronchospasm (bronchospasm).
Tell your doctor or pharmacist if any of the side effects you listed
or you notice any side effects not listed in this leaflet.5. How to keep codein?
Store drug out of reach of children.
Do not store above 25 ° C.
This medicine is stable for 6 months after onset.
You may take the medicinal product according to the expiry date stated on the packaging and on the label.
Do not use the date.
The medicinal product must not be disposed of in waste water or household waste. Ask your
Pharmacist how to dispose of the medicine when you no longer need it. These measures
helps to protect the environment. -
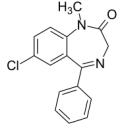
Diazepam
1. What is Diazepam and what is it used for?
Diazepam is a medicine used to treat anxiety and tension in the benzodiazepine group.
Diazepam ratiopharm® 5 mg is used
for the symptomatic treatment of acute and chronic tension, agitation and anxiety. The use of Diazepam in sleep disorders requiring treatment, which are caused by anxiety, tension and arousal, is only justified if at the same time the diazepam effects are desired during the daytime. Note: Not all states of tension, excitation and anxiety require drug therapy. Often they are expressions of physical or mental illness and can be influenced by other measures or by a therapy of the underlying disease.
for reassurance and preparation (premedication) before surgical and diagnostic interventions or afterwards (postoperative medication).
for the treatment of conditions with increased muscle tension (increased muscle tone).2. What do you need to know before you take Diazepam?
Diazepam should not be taken
if you are allergic (hypersensitive) to diazepam or other benzodiazepines or any of the other ingredients of Diazepam ratiopharm 5 mg
Dependent disease in history (alcohol, drugs, drugs)
in certain forms of severe, pathological muscle weakness (myasthenia gravis)
Take special care with Diazepam
acute poisoning with alcohol, sleeping and painkillers as well as preparations for the treatment of mental and emotional disorders (neuroleptics, antidepressants and lithium)
Disorders of the ordered interaction of muscle groups (spinal and cerebellar ataxias)
acute increase in intraocular pressure (narrow-angle glaucoma, cataract)
severe liver damage, eg. B. Jaundice with biliary congestion (cholestatic jaundice)
Respiratory dysfunction in sleep (sleep apnea syndromes)
Patients with organic brain changes
Circulatory and respiratory weakness (chronic obstructive respiratory insufficiency)
Prolonged intake is recommended for blood and liver function.
This medicine contains a "benzodiazepine". Benzodiazepines are medicines used to treat certain conditions associated with restlessness, anxiety, inner tension or insomnia. When using benzodiazepines there is a danger of training or promoting dependency. Even with daily intake over a few weeks, the danger of a dependency development is given. This applies not only to the misuse of particularly high doses, but also to the therapeutic dose range. To minimize this risk, you are advised to pay close attention to the following:
Benzodiazepines have been created solely for the treatment of morbid conditions and may only be taken on medical advice.
At the latest after 4 weeks of taking the doctor should decide whether a treatment must be continued. Continuous, longer-term intake should be avoided as it can lead to dependency. Taking it without a doctor's prescription reduces your chances of helping you with these medicines.
Never increase the dose prescribed by the doctor, even if the effect wears off. By unauthorized dose increase, the targeted treatment is difficult.
When discontinued after prolonged use, restlessness, anxiety and insomnia can occur, often with a few days delay. These settling symptoms generally disappear after 2-3 weeks.
If you are or have ever been addicted to alcohol, medicines or drugs, you should not take benzodiazepines; rare, only situations to be judged by the doctor. Tell your doctor about this.
Never take medicines containing benzodiazepines because they have "helped others so well" and never give them to others.Taking Diazepam with other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. Concomitant use of other drugs acting on the nervous system may increase the effects of each other. These medicines include, for. B. preparations for the treatment of mental disorders (psychotropic drugs), sleeping pills, some painkillers, anesthetics or certain drugs for the treatment of allergies or colds (antihistamines).
The effect of medicines that lower muscle tension (muscle relaxants) can be strengthened.
If you are taking cimetidine or omeprazole (medicines used to treat stomach ulcers, for example) or disulfiram (medicines used to treat alcohol withdrawal), the effects of Diazepam may be increased and prolonged.
In smokers, the excretion of diazepam can be accelerated.
Theophylline (medicines used to treat, for example, asthma) in low doses relaxes Diazepam sedation.
Diazepam may inhibit the effects of levodopa (Parkinson's disease medicines).
In rare cases, Diazepam can inhibit the metabolism of phenytoin (an epilepsy drug) and increase its effectiveness. Phenobarbital and phenytoin (medicines used to treat epileptic seizures) can accelerate the metabolism of Diazepam.
In patients undergoing long-term treatment with other medicinal products, such as For example, certain medicines for high blood pressure (centrally effective antihypertensive agents, beta-adrenoceptor blockers), anticoagulants and cardiovascular agents (cardioactive glycosides) are unpredictable in their nature and extent. Therefore, special care should be taken when taking the product at the same time, especially at the beginning of treatment. Tell your doctor if you are receiving such long-term treatment.
Due to slow excretion of diazepam from the body, you should still expect possible interactions after stopping treatment with Diazepam.
Taking Diazepam with food and drink
Avoid alcohol during treatment with Diazepam, as alcohol will alter and enhance the effects of Diazepam unpredictably.
pregnancy and breast feeding period
In pregnancy, you should only use Diazepam in exceptional cases for compelling reasons. If you become pregnant or suspect you are pregnant while receiving Diazepam-ratiopharm®, tell your doctor immediately. Long-term use of Diazepam during pregnancy may lead to withdrawal symptoms in the newborn. The administration of larger doses before or during childbirth can cause a lowered body temperature, depressed respiration, reduced muscle tension and dehydration (so-called "floppy-infant syndrome") in the infant.
During breast-feeding, you should not use Diazepam as diazepam, the active substance in Diazepam, and its breakdown products are excreted in breast milk. If treatment is inevitable, it should be weaned.
Driving and using machines
This medicinal product, even when used properly, may alter the ability to react so far as to affect the ability to drive or operate machinery. This applies even more in combination with alcohol.
Therefore, you should refrain from driving, operating machinery or any other dangerous activity altogether, or at least during the first few days of treatment. The decision in each individual case is made by the treating physician, taking into account the individual reaction and the respective dosage.
Important information about some of the ingredients of Diazepam
This medicine contains lactose. Therefore, you should only take Diazepam after consulting your doctor, if you are aware that you have an intolerance to certain sugars.3. How to take Diazepam?
Always take Diazepam exactly as your doctor has told you. Please check with your doctor or pharmacist if you are not sure.
The dosage depends on the individual reaction situation, age and weight of the patient as well as the type and severity of the clinical picture. The principle is to keep the dose as low and the duration of treatment as short as possible.
Unless otherwise prescribed by the doctor, the usual dose is
Treatment-requiring tension, excitement and anxiety
Out-of-hospital treatment Adults and adolescents over the age of 14 take 1 tablet once daily or ½ tablet twice daily (equivalent to 5 mg diazepam per day) at the beginning of treatment. If at this dose the desired effects can not be achieved, the dose may be increased by the doctor twice daily 1 tablet or once daily 2 tablets (equivalent to 10 mg diazepam per day). Higher doses are only necessary in rare cases (eg psychiatric and neurological diseases) and are usually only in the hospital.
Inpatient treatment In severe tension, agitation, anxiety and restlessness, the dose may be gradually increased to 3 to 6 times daily 2 tablets (equivalent to 30-60 mg diazepam per day). Dosage strengths of 10 mg are also suitable for this purpose.
Treatment of conditions with increased muscle tension
At the beginning of the treatment, take 1 tablet (equivalent to 10-20 mg diazepam per day) 2 to 4 times a day. To continue treatment, 1 tablet (equivalent to 5-10 mg diazepam per day) is sufficient once or twice daily.
For reassurance and surgical preparation in anaesthesiology and surgery or afterwards (premedication / postoperative medication)
On the eve of surgery adults receive 2-4 tablets (equivalent to 10-20 mg diazepam). After the operation Adults receive 1-2 tablets (equivalent to 5-10 mg diazepam), if necessary a repetition is possible.
Special dosage instructions
Elderly or weakened patients as well as patients with organic brain changes, circulatory and respiratory dysfunction as well as impaired liver or kidney function as well as children over the age of 3 and adolescents up to the age of 14 generally receive half of the daily dosage indicated above; H. initially ½ tablet per day (equivalent to 2.5 mg diazepam) to a maximum of 1 tablet (5 mg diazepam).
type of application
Take Diazepam whole with plenty of fluid. The tablets are divisible. For outpatient treatment of tension, agitation and anxiety, you should take Diazepam mainly in the evening.
In the evening you should take Diazepam about half an hour before going to bed and not on a full stomach, otherwise with delayed onset of action and - depending on the sleep duration - with increased after effects (eg, tiredness, difficulty concentrating) next Morning must be expected.
For inpatient treatment of tension, excitement and anxiety, as well as for the treatment of conditions of increased muscle tension, you should take Diazepam throughout the day regardless of the meals.Duration of application
Depending on the type and severity of the disease, the doctor will decide on the duration of the dose.
In acute tension, agitation and anxiety, you should limit the use of Diazepam to single doses or a few days.
In chronic tension, agitation and anxiety, the duration of ingestion depends on the course. After 2 weeks of daily use, the doctor should determine by a gradual dose reduction if further treatment with Diazepam is indicated. However, you should not use Diazepam for more than 4 weeks in chronic tension, agitation and anxiety.
For prolonged periods of use (longer than 1 week), the dose should be gradually reduced when discontinuing Diazepam. You must be aware of possible withdrawal symptoms (see "If you stop taking Diazepam ratiopharm® 5 mg").
For the preparation of surgical or diagnostic procedures, as well as for the treatment of conditions with increased muscle tension, the drug is generally used at short notice.
If you have the impression that the effect of Diazepam is too strong or too weak, talk to your doctor or pharmacist.
If you take more Diazepam ratiopharm 5 mg than you should
In case of overdose, the doctor should ask for advice. Regardless, you can try to empty the stomach contents by forced vomiting. Each time a poisoning is assessed, consideration should be given to multiple poisoning by possible ingestion / use of multiple drugs. The symptoms of overdose are more pronounced under the influence of alcohol and other brain depressants.
Symptoms of overdose and necessary action
Symptoms of mild overdose can be Confusion, drowsiness, gait and movement disorders, slurred speech, drop in blood pressure, muscle weakness. If such signs of disease appear, a doctor should be informed immediately, who decides on the severity and any further measures that may be required.
In cases of severe poisoning, central reduction of cardiovascular and respiratory functions may occur with blue-red staining of the skin and mucosa, unconsciousness or even respiratory arrest or cardiac arrest. In such cases intensive monitoring is necessary!
High levels of arousal may occur during the decay phase.
If you forget to take Diazepam
Take the prescribed dose at the next scheduled time, but never twice the dose.
If you stop taking Diazepam
If you want to stop treatment, talk to your doctor first. Do not terminate the medical treatment without your doctor's advice. You can endanger the success of therapy.
By sudden discontinuation of the drug after prolonged daily use may occur after about 2-4 days insomnia and increased dreams. Anxiety, states of tension as well as arousal and inner restlessness can get more intensified. The appearance can manifest itself in tremors and sweating and may increase to threatening physical (eg, convulsions) and mental reactions such as symptomatic psychosis (eg withdrawal delirium).
If you have any further questions on the use of the medicine, ask your doctor or pharmacist.4. Side effects of diazepam
Frequently
Unwanted strong calm during the day as well as fatigue (drowsiness, fatigue, drowsiness, prolonged reaction time), dizziness, gait and movement disorders, headache, confusion; In addition, temporary memory lapses may occur after taking diazepam.
Concentration disorders and residual fatigue may affect responsiveness in the morning after evening administration.
Due to the pronounced muscle-relaxing effect of Diazepam, caution (risk of falling!) Is required especially in the elderly.
Rare
gastrointestinal upset (nausea, vomiting, abdominal discomfort, constipation, diarrhea), jaundice, urinary retention, fits of cramps, chest pain, drop in blood pressure, slowing of heartbeat, depression, decreased sexual desire, and in menstruation disorders , Appetite increase, dry mouth, allergic skin changes (such as itching, redness, rash) as well as respiratory damping.
The atraumatic effect may be more pronounced in the case of existing respiratory distress due to narrowed airways and in patients with brain damage. This is especially important when used with other medicines that affect the brain.
Excessive doses of the drug for several days may cause colicky abdominal pain and diarrhea.
High doses and prolonged use of Diazepam may cause temporary disturbances such as slow or slurred speech, visual disturbances (double vision, blurred vision, eye shaking), movement and gait insecurity.
Prolonged or repeated use of Diazepam may lead to a decrease in the effect (so-called tolerance development).
In patients with pre-existing depressive illness depressive moods can be exacerbated. There is also the possibility that sensory disorders (hallucinations) occur or an inversion of activity ("paradoxical reactions"), such. As acute arousal states, anxiety, suicidality (suicidal tendencies), sleep disorders, tantrums or increased muscle spasms occurs.
Weaning phenomena: See 3. under "If you stop taking Diazepam ratiopharm® 5 mg".
countermeasures
If you experience one or more of the above side effects, tell your doctor so that he or she can decide on the severity and any other necessary measures. Side effects generally recur after dose reduction and can usually be avoided by careful and individual adjustment of daily doses.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.5. How to store Diazepam?
Store drug out of reach of children.
Do not use after the expiry date which is stated on the carton and blister packs. The expiration date refers to the last day of the month.
This medicinal product does not require special storage conditions. -
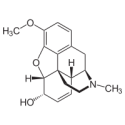
Fentanyl
What is fentanyl?
The name of your medicine is Fentanyl.
The Pfl
Aster help, strong and long-lasting pain
to treat:
• in adults who have a continuous pain
need action
• in children over 2 years of age who are already receiving opioids
and need continuous pain management
Fentanyl contains an active substance called fen-
tanyl. This belongs to a group more effective
Painkillers - called opioids.What to care about when I take fentanyl?
Fentanyl must not be used
be if you
• allergic to fentanyl, hydrogenated rosin,
Soy, peanut or any of those mentioned in section 6
ingredients of this medicine.
• have pain that last only a short time, like
sudden onset of pain or pain
an operation.
• breathing problems with slower and fl
have breathing.
Do not use this medicine if any of the
mentioned above applies to you or your child. If they
are not sure, talk before using fentanyl
with your doctor or pharmacist.How to take fentanyl?
Always use this medicine exactly as you
speak with your doctor. Ask your doctor or
Pharmacist after, if you are not sure.
Depending on the severity of your pain, your overall
general condition and the type of pain
therapy, your doctor will decide what strength of fentanyl
is best for you.
Application and change of plaster
• There is enough active ingredient in each patch
3 days (72 hours)
contain.
• You should change your patch every third day, except
Your doctor has told you otherwise.
• Always remove the old plaster first,
before You
stick a new one on.
• Always change your plaster every 3 days
at the same
daytime
(every 72 hours).
• If you use more than one patch, switch
all plaster at the same time.
• Make a note about the day of the week, the
Date and time you stuck a plaster
have to remember changing the plasterSide effects of fentanyl?
Like all medicines, this medicinal product
have effects that do not have to happen to everyone.
If you or your partner or caregiver has any of the following about the person doing the
Plaster wears, notice, remove the plaster
and call a doctor immediately or go
directly to the nearest hospital. You can
urgently need medical treatment.
• abnormal feeling of drowsiness, a slower one
or flatter breathing than usual
Follow the instructions above and
hold the person who has been wearing the patch
as much as possible to move and speak. Very sel-
These breathing difficulties can be life-threatening
or even fatal, especially in persons who
So far, no strong opioid painkillers (such
Fentanyl or morphine).
ben. (Occasionally, this can affect up to 1 in 100
concern)
• sudden swelling of the face or throat,
severe irritation, redness or blistering of your skin
These may be signs of severe allergic reactions.
(the frequency is calculated on the basis of the available
can not be estimated).How to store fentanyl?
Where should the patches be kept?
Keep all plaster (used and unused) for children
the inaccessible.
How long can Fentanyl be stored?
You may use Fentanyl after
box and the bag after "usable until"
do not use the expiration date. The decay
Date refers to the last day of the specified
Month. When the patches have expired, bring them
back to the pharmacy.
Storage conditions
Store in the original packaging.
How to dispose of used or unused patches
If a used or unused plaster accidentally
is liable to another person, especially one
Child, this can be deadly.
Used patches should be folded firmly in the middle,
so that the glue
cling together. Then you should
put them back in the original bag and for others
Persons, especially children, kept out of reach
until they are safely disposed of.
Ask your pharmacist how to use the medicine.
Worry is when you stop using it.
Do not dispose of medicines in wastewater or household
stop falling. You help to protect our environment. -
Buprenorphine
Buy Buprenorphine online
Buprenorphine (Subutex®, Buprenorphine mepha®)
Buprenorphine (Subutex®, buprenorphine mepha®) may offer advantages over methadone in certain situations for pharmacological reasons. Basically, clarification / status and approval procedures are the same as with methadone, see "SBG: Start in 1 Consultation" (best practice, suitable for experienced doctors) or "SGB Start in 2 Consultations". An aid for the differentiated indication for buprenorphine can be found here. Buprenorphine is much more expensive than methadone, but is also a compulsory health insurance.
Pharmacokinetics
Sublingual administration with rapid absorption (pronounced first-pass effect, therefore hardly bioavailable after swallowing and low risk of intoxication). After 3-5 minutes the tablets dissolve under the tongue (to fully allow the effect of the tablets, do not swallow for 5 minutes).
sublingual bioavailability = 31-52%
Time to reach a clinical effect approx. 30 (-60) minutes
Time to reach maximum plasma levels about 90-150 minutes
Time to reach maximum clinical effect about 1-4 hours
Rapid absorption from the plasma and redistribution into various tissues (e.g., adipose tissue). Out of the different tissues, there is a time-delayed, slow redistribution, which ensures constant buprenorphine plasma levels ("depot effect"). Effective plasma levels: between 0.7 and 12 ng / ml; dose-dependent duration of action Steady state: about after 5-8 days; no significant daily fluctuations with regular intake. Most of the metabolism is via cytochrome P450 3A4. Since other enzymes are involved in the degradation of buprenorphine (CYP 2C8), the degradation metabolism of buprenorphine and its metabolites is relatively insensitive to interactions. Elimination half-life: 20-25 / 37hours. The elimination is predominantly hepatic by glucuronidation and N-dealkylation. The excretion is about 70-80% of the faeces, the rest, 20-30%, renal.Mechanism of action
Buprenorphine is a partial agonist on the mu opioid receptor (mediating effects such as euphoria, analgesia, respiratory depression and dependence) and an antagonist on the kappa receptor (mediating effects such as dysphoria and sedation). Compared to methadone and heroin, buprenorphine shows important differences due to its special properties at the opioid receptors: Buprenorphine has a higher receptor affinity (heroin and methadone are displaced) and a moderate intrinsic agonistic activity with only partial stimulation of the mu opioid receptors (high doses of buprenorphine result in a lighter, less euphoric and less sedating central nervous effect than high doses of other opioids such as heroin, methadone). Ceiling Effect: There is no linear dose-response relationship. With increasing dosage it comes to reaching a Wirkplateaus. Buprenorphine, in combination with other central depressants, may contribute to a limited respiratory depressive effect. Buprenorphine shows a long receptor half-life with slow receptor dissociation and less receptor-down regulation.